Questions 7-12 are based on the following passage.
Aluminum water-based paints (AWPs) contain aluminum (Al) flakes that give surfaces a shiny, metallic appearance. If the flakes corrode, a dull coating of aluminum hydroxide forms on them:
2Al+6H₂O⟶2Al(OH)₃+3H₂
Table 1 shows the volume of $$H_2$$ gas produced over time (at 25°C and 1 atm) from 100 mL samples of freshly made AWPs 1-3 in 3 separate trials. AWPs 1-3 were identical except that each had a different concentration of DMEA, an AWP ingredient that increases pH. 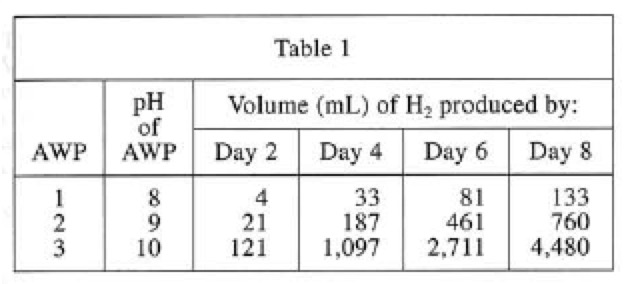
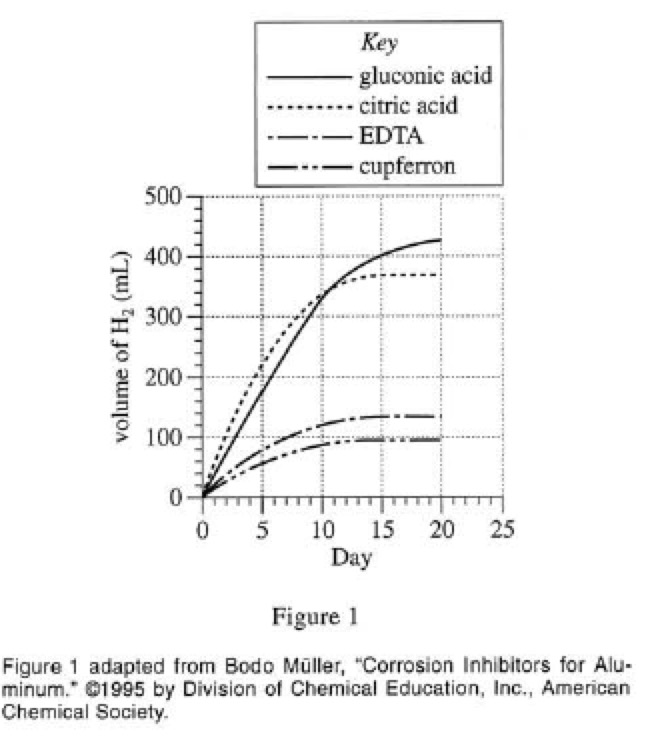
The AWP 3 trial was repeated 4 times, but for each trial, the sample had the same concentration of 1 of 4 corrosion inhibitors (see Figure 1).