Questions 1-11 are based on the following
passage.
This passage is excerpted from Douglas Fox, “Primordial Soup’s On: Scientists Repeat Evolution’s Most Famous Experiment” © 2007 by Scientific American.
A Frankensteinesque contraption of glass bulbs and
crackling electrodes has produced yet another revelation
about the origin of life.
Line The results suggest that Earth's early atmosphere could
5 ave produced chemicals necessary for life-contradicting
the view that life's building blocks had to come from comets
and meteors. "Maybe we're over-optimistic, but I think this is
a paradigm shift," says chemist Jeffrey Bada, whose team
performed the experiment at the Scripps Institution of
10 Oceanography in La Jolla, California.
Bada was revisiting the famous experiment first done by
his mentor, chemist Stanley Miller, at the University of
Chicago in 1953. Miller, along with his colleague Harold
Urey, used a sparking device to mimic a lightning storm on
15 early Earth. Their experiment produced a brown broth rich in
amino acids, the building blocks of proteins. The disclosure
made the pages of national magazines and showed that
theories about the origin of life could actually be tested in the
laboratory.
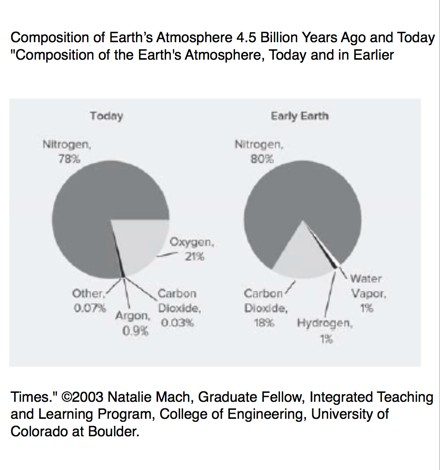
20 But the Miller-Urey results were later questioned: It turns
out that the gases he used (a reactive mixture of methane and
ammonia) did not exist in large amounts on early Earth.
Scientists now believe the primeval atmosphere contained an
inert mix of carbon dioxide and nitrogen-a change that
25 made a world of difference.
When Miller repeated the experiment using the correct
combo in 1983, the brown broth failed to materialize.
Instead, the mix created a colorless brew, containing few
amino acids. It seemed to refute a long-cherished icon of
30 evolution-and creationists quickly seized on it as supposed
evidence of evolution's wobbly foundations.
But Bada's repeat of the experiment-armed with a new
insight-seems likely to turn the tables once again.
Bada discovered that the reactions were producing
35 chemicals called nitrites,which destroy amino acids as
quickly as they form.They were also turning the water acidic
-which prevents amino acids from forming. Yet primitive
Earth would have contained iron and carbonate minerals that
neutralized nitrites and acids. So Bada added chemicals to the
40 experiment to duplicate these functions. When he reran it, he
still got the same watery liquid as Miller did in 1983, but this
time it was chock-full of amino acids.
"It's important work," says Christopher McKay, a
planetary scientist at NASA Ames Research Center in
45 Moffett Field, Calif. "This is a move toward more realism in
terms of what the conditions were on early Earth."
Most researchers believe that the origin of life depended
heavily on chemicals delivered to Earth by comets and
meteorites. But if the new work holds up, it could tilt that
50 equation, says Christopher Chyba, an astrobiologist at
Princeton University. "That would be a terrific result for
understanding the origin of life," he says, "and for
understanding the prospects for life elsewhere."
But James Ferris, a prebiotic chemist at Rensselaer
55 Polytechnic Institute in Troy, N.Y., doubts that atmospheric
electricity could have been the only source of organic
molecules. "You get a fair amount of amino acids," he says.
"What you don't get are things like building blocks of nucleic
acids." Meteors, comets or primordial ponds of hydrogen
60 cyanide would still need to provide those molecules.
Bada's experiment could also have implications for life on
Mars, because the Red Planet may have been swaddled in
nitrogen and carbon dioxide early in its life. Bada intends to
test this extrapolation by doing experiments with lower-
65 pressure mixes of those gases.